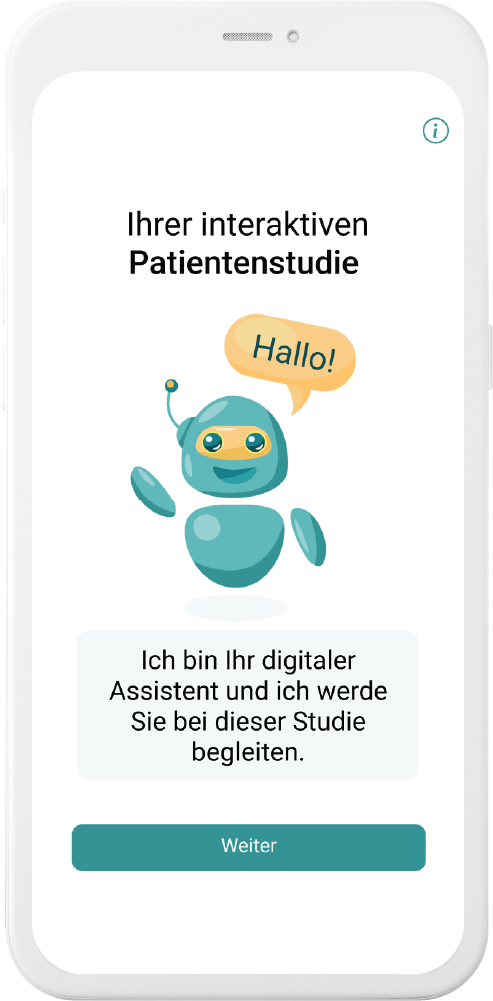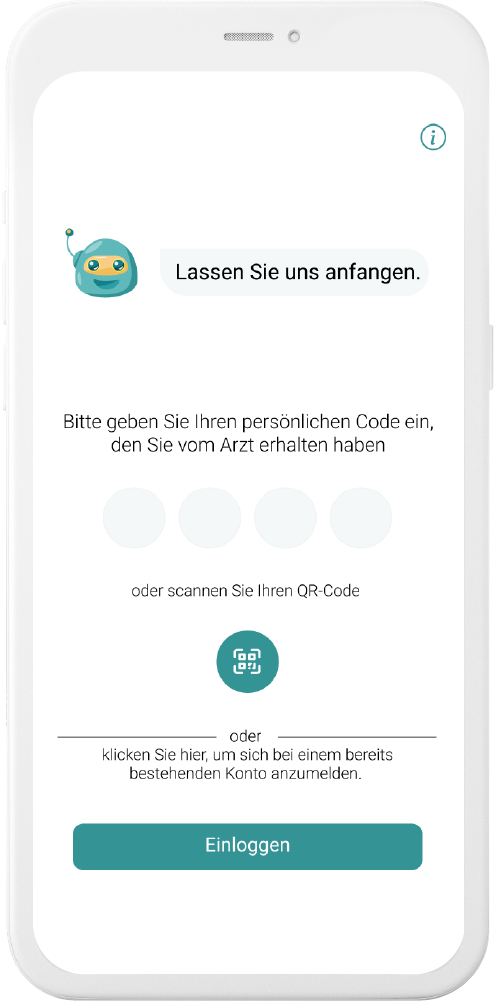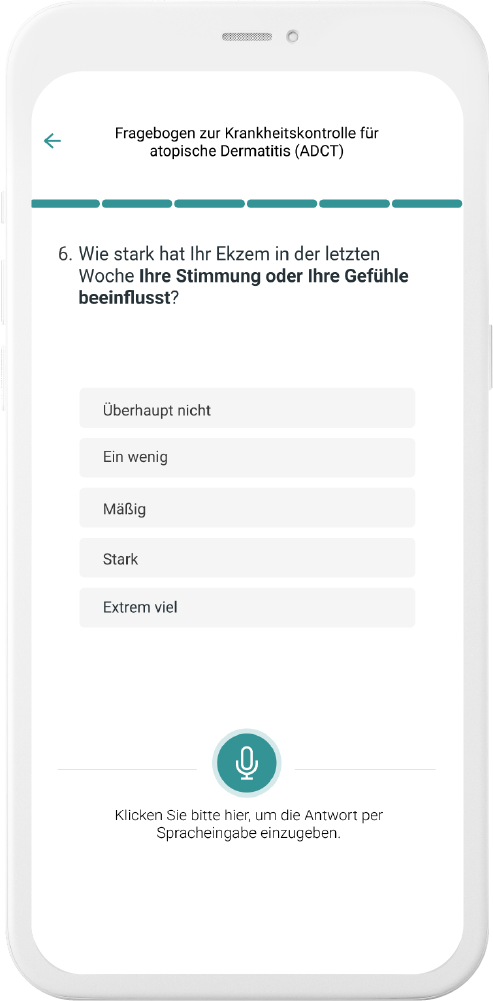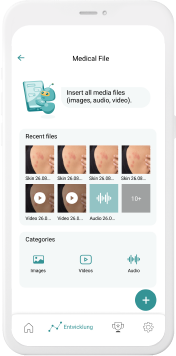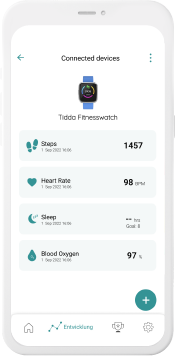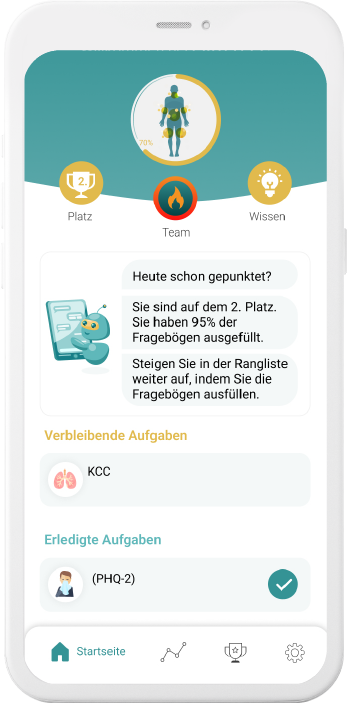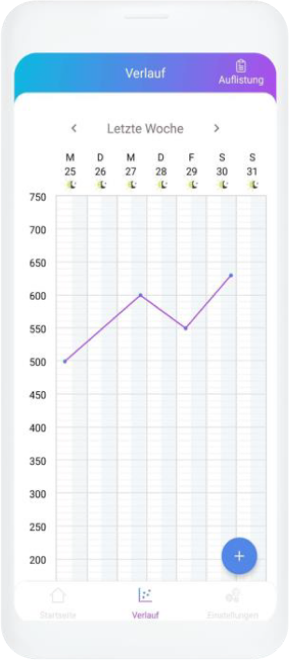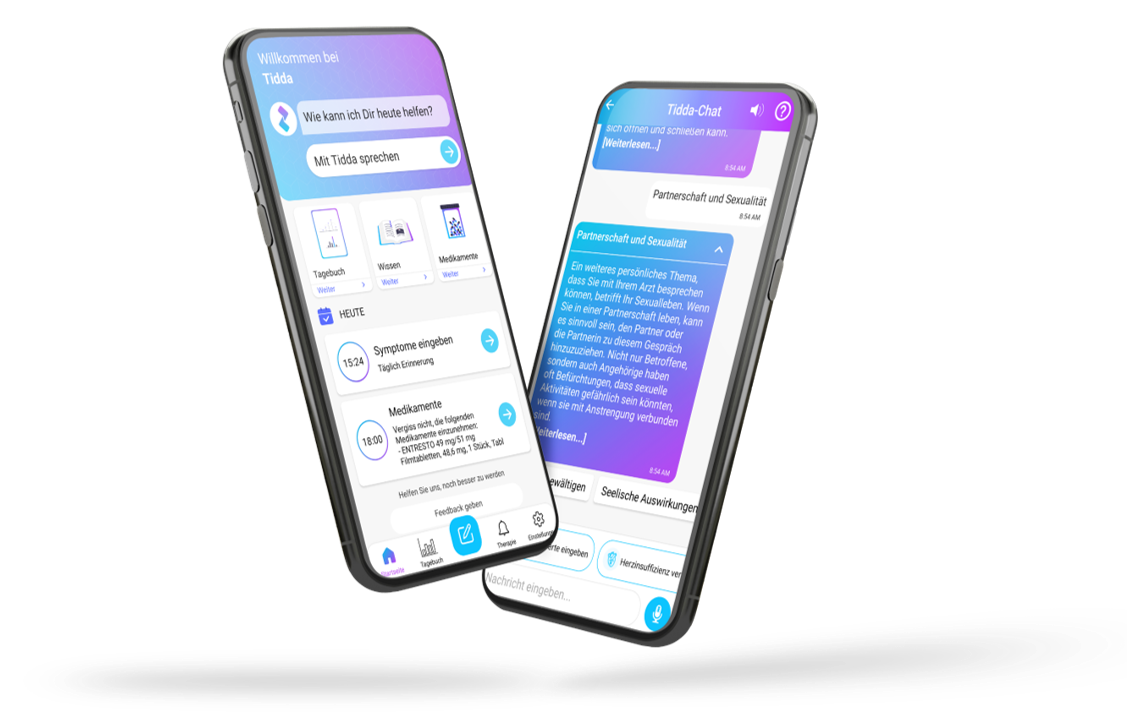
Our digital patient companion offers a cost-effective and scalable tool to remotely engage patients in clinical trials.

Medical-Device Certification

GDPR Compliant

Data Anonymization

Secure Encryption

Made in Germany

Expand real-world evidence
70% of potential participants live over 2 hours away from trial sites¹, while the average willingness to travel is less than 30 minutes².
Our digital companions integrated in everyday devices help to reach more patients at scale, anywhere and anytime.

Improve compliance & retention
Systematic data for clinical trials show 24% patients are non-adherent and 12% suboptimal adherent.
Our digital companions motivate with empathic and personalizedinteractions to comply better and stay longer on trial.

Save cost & time
Adequate number of participants and study center visits are the largest single factors driving study costs.
Our patient apps collect data directly from patients, bypassing costly study center visits. They increase engagement reducing the risk of dropouts.
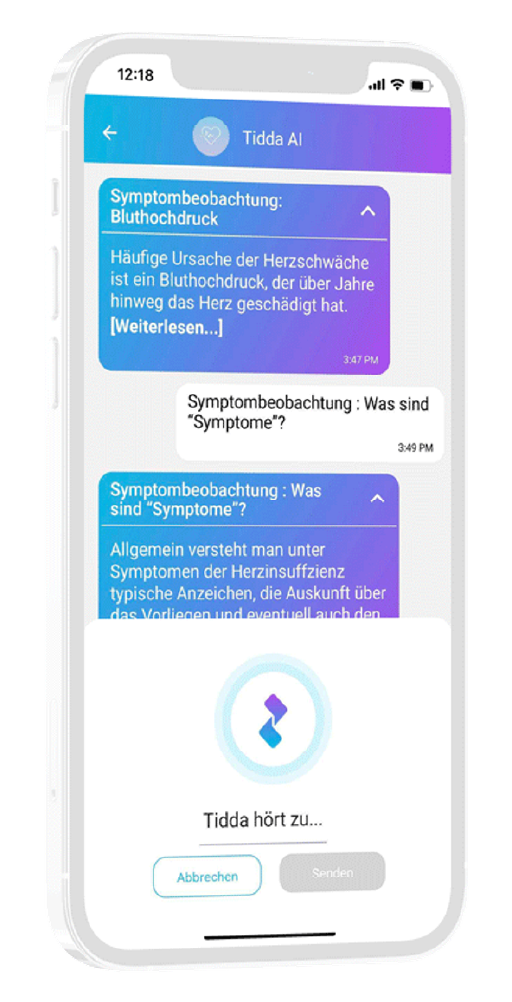

Interaction through natural language
User can interact with in-app companion through text and voice communication
Personalization
Patient receives interactive and personalized information onstudy and indication
Data generation is quick and easy
User can easily fill out questionnaires and report data in chat or with voice
Tech expertise & regulatory compliance
- Innovative technology to empower patient engagement and interaction
- Tested and proven: implemented in several clinical trials
- Compliance with Ethical Committee procedures following best practices

Safe & secure
- Safety-first and privacy by design
- CE-certified medical device MDD Class I
- Software built in-house
- Data storage in Germany only

Integration & customization
- Safety-first and privacy by design
- CE-certified medical device MDD Class I
- Software built in-house
- Data storage in Germany only
Our Partner





100 percent of pharma companies and CROs expect virtual trials to be a major component of their portfolio, 89 percent expect to run a trial with most activities conducted in participants' homes.
(McKinsey study 2021)